Diazo chemistry
Fluorine chemistry
Fluorine in metal-catalyzed asymmetric transformations: the lightest halogen causing a massive effect
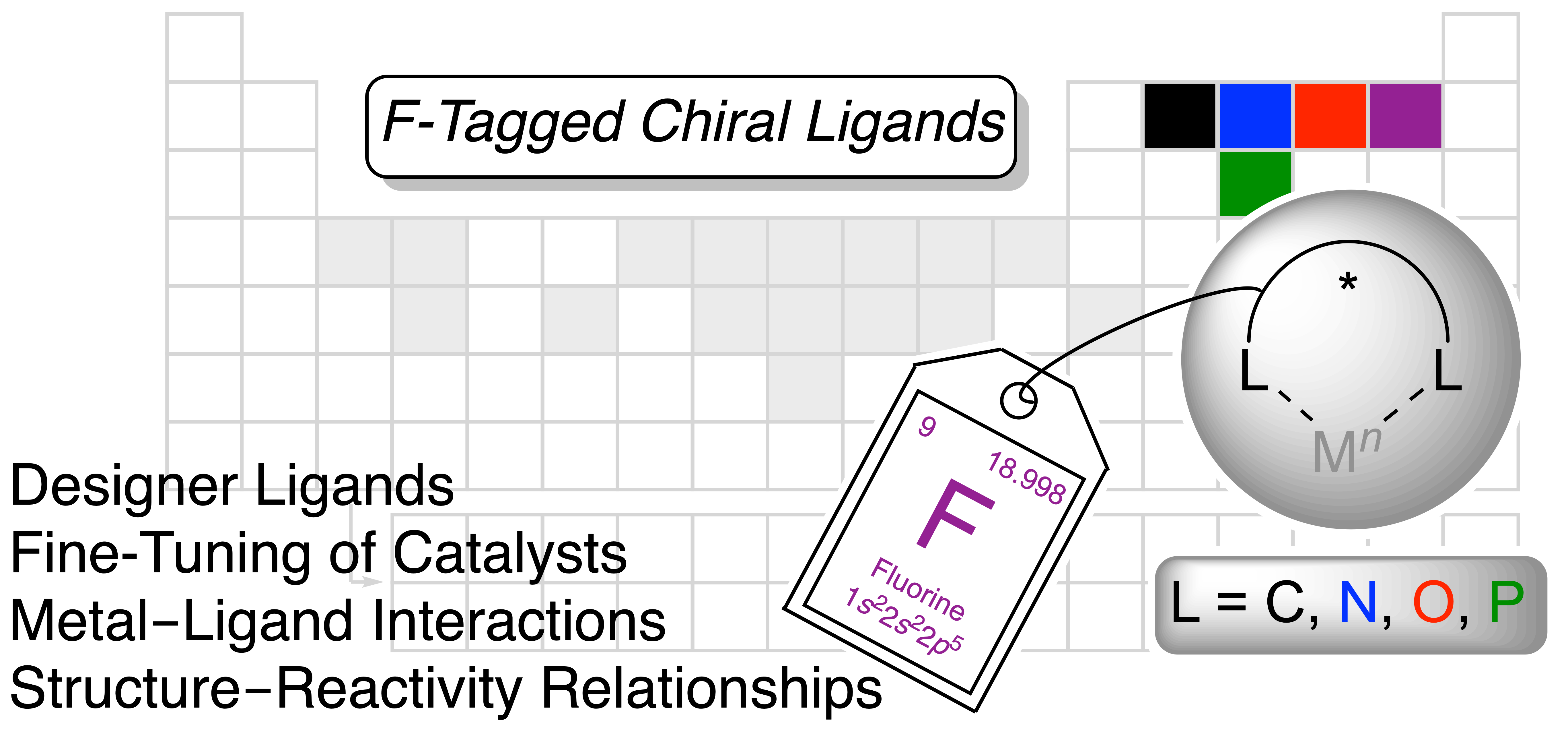
Lauzon, S.; Ollevier, T. Chem. Sci. 2022, in press. doi: 10.1039/d2sc01096h.
Emerging Applications of Aryl Trifluoromethyl Diazoalkanes and Diazirines in Synthetic Transformations
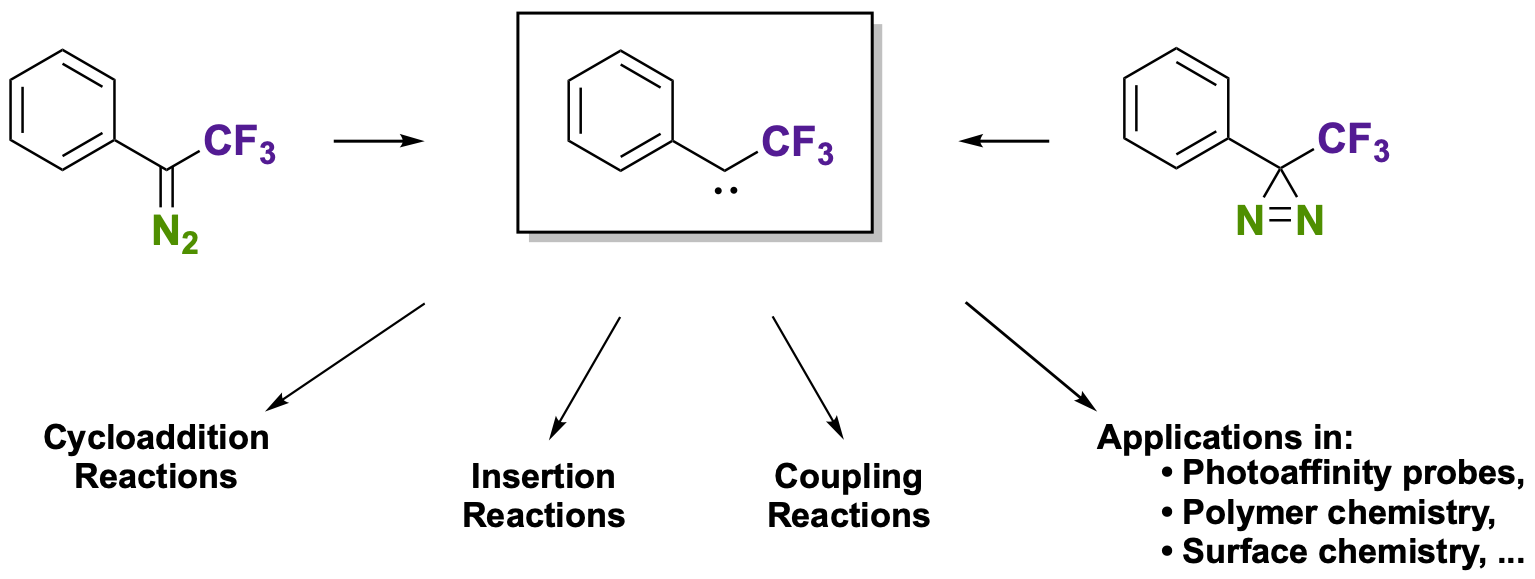
Ollevier, T.; Carreras, V. ACS Org. Inorg. Au 2022, in press. doi: 10.1021/acsorginorgau.1c00027.
Fluoride-triggered Synthesis of 1-Aryl-2,2-Difluoroalkenes via Desilylative Defluorination of (1-Aryl)-2,2,2-trifluoroethyl-silanes
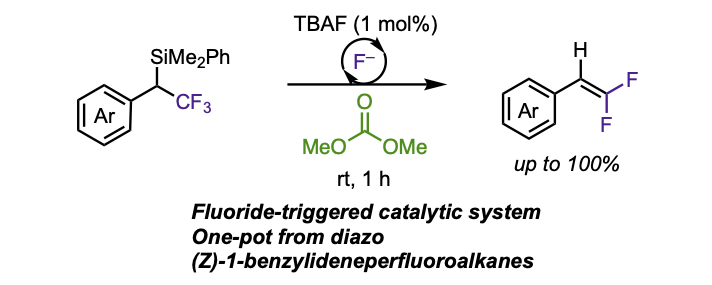
Carreras, V.; Ollevier, T. J. Org. Chem. 2021, 86, 13160–13168. doi: 10.1021/acs.joc.1c01724.
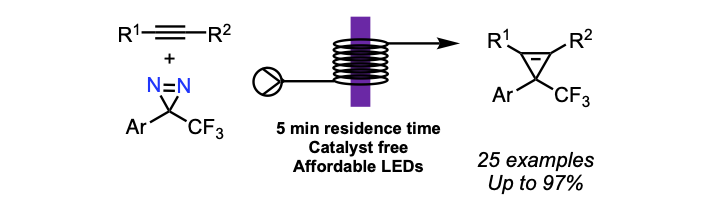
Tanbouza, N.; Carreras, V.; Ollevier, T. Org. Lett. 2021, 23, 5420–5424. doi: 10.1021/acs.orglett.1c01750.
Asymmetric CuI-Catalyzed Insertion Reaction of 1-Aryl-2,2,2-trifluoro-1-diazoethanes into Si–H Bonds
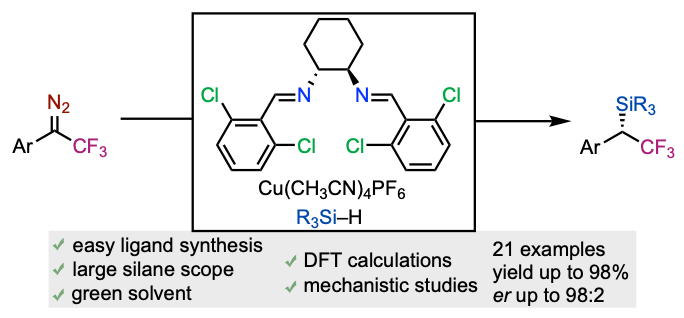
Carreras, V.; Besnard, C.; Gandon, V; Ollevier, T. Org. Lett. 2019, 21,9094–9098. doi: 10.1021/acs.orglett.9b03480.
Iron chemistry
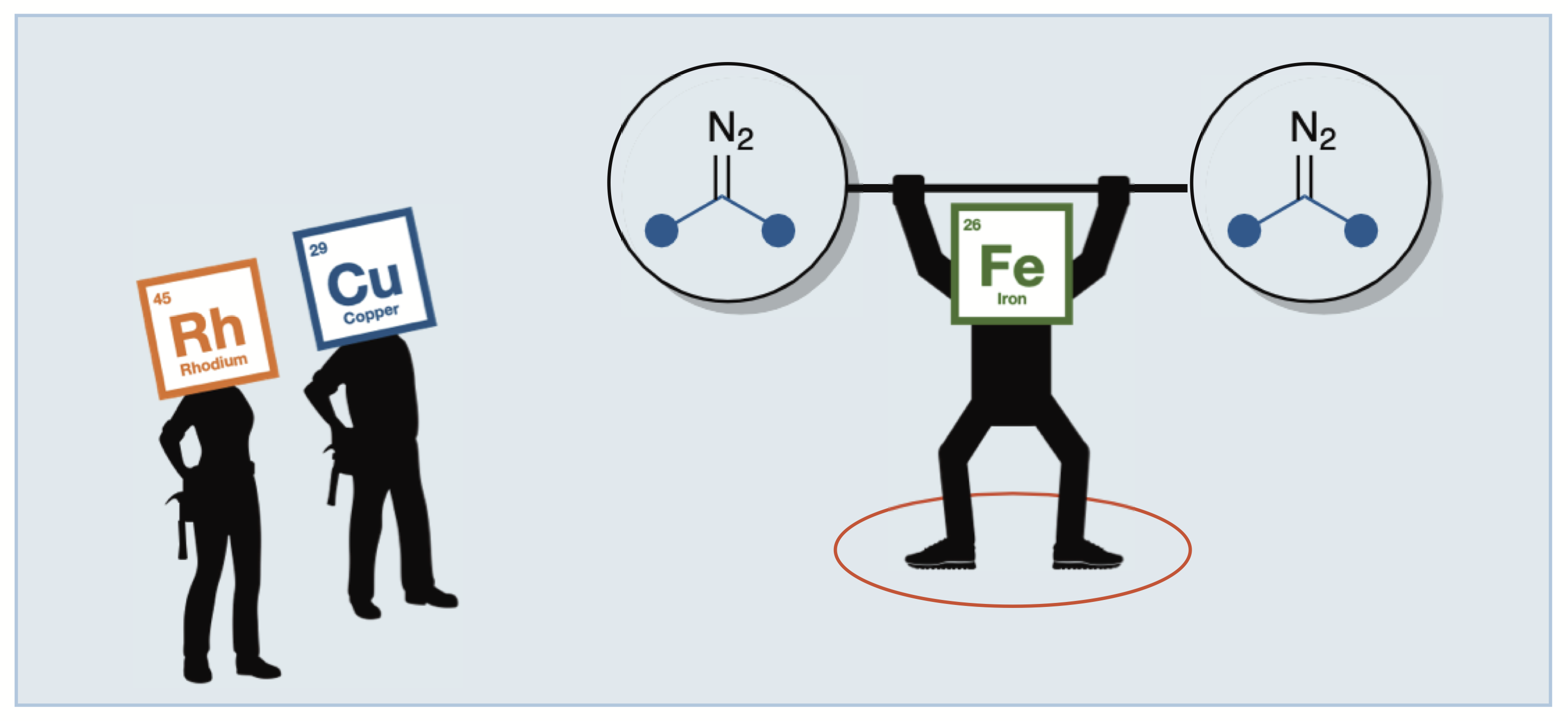
Carreras, V.; Tanbouza, N.; Ollevier, T. Synthesis 2021, 53,79–94. doi: 10.1055/s-0040-1707272. This article is editor's choice.
FeII-Catalysed Insertion Reaction of α-Diazocarbonyls into X–H Bonds (X = Si, S, N, and O) in Dimethyl Carbonate as a Suitable Solvent Alternative
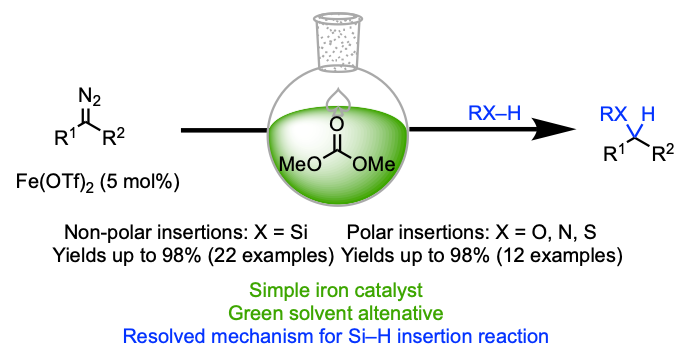
Tanbouza, N.; Keipour, H.; Ollevier, T. RSC Advances 2019, 9, 31241–31246. doi: 10.1039/c9ra07203a.
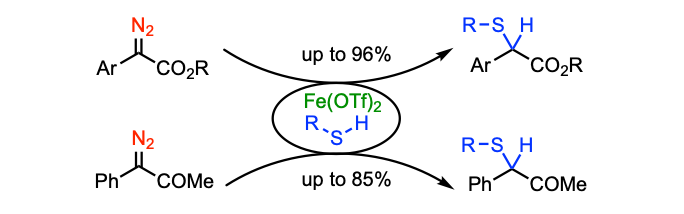
Keipour, H.; Jalba, A.; Tanbouza, N.; Carreras, V.; Ollevier, T. Org. Biomol. Chem. 2019, 17, 3098–3102. This article is part of the themed collections: "Synthetic methodology in OBC" and "Carbenes in Organic Synthesis". doi: 10.1039/c9ob00261h.
Copper chemistry
Copper-Catalyzed Carbenoid Insertion Reactions of α-Diazoesters and α-Diazoketones into Si–H and S–H Bonds
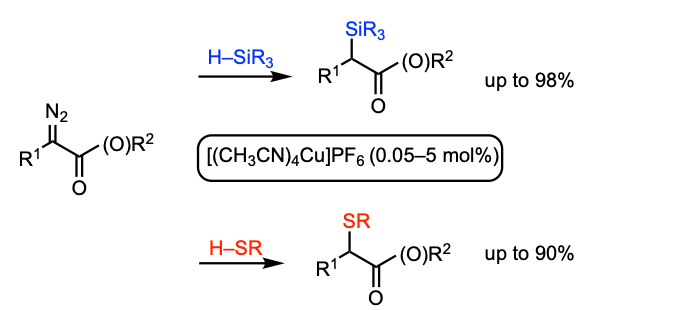
Keipour, H.; Jalba, A.; Delage-Laurin, L.; Ollevier, T. J. Org. Chem. 2017, 82, 3000–3010. doi: 10.1021/acs.joc.6b02998.
Asymmetric catalysis
Ligand design
C2-Symmetric 2,2'-Bipyridine-α,α'-1-adamantyl-diol Ligand: Bulky Iron-Complexes in Asymmetric Catalysis
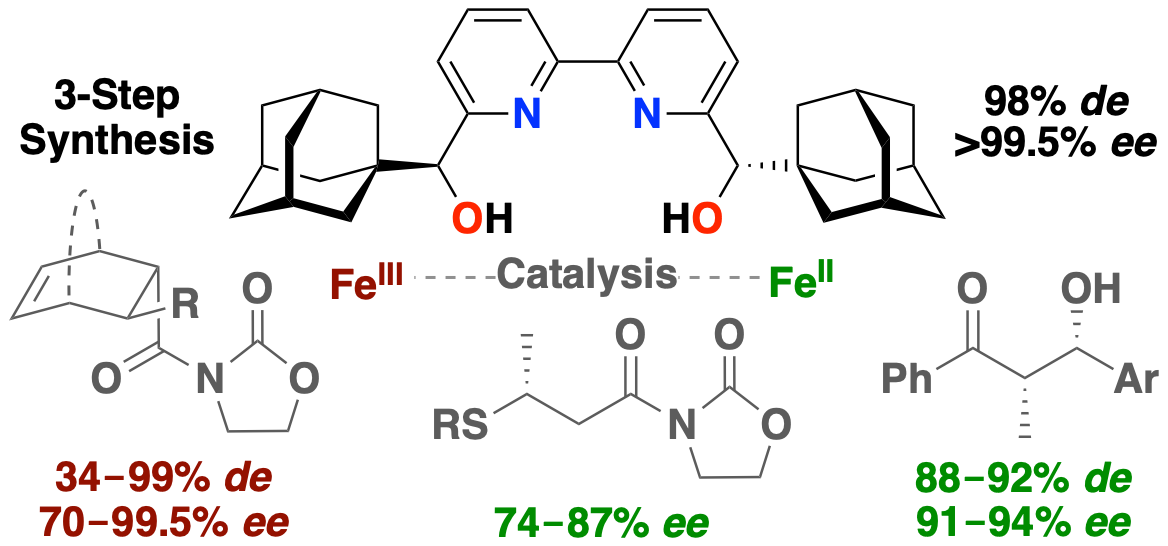
Lauzon, S.; Ollevier, T. Org. Lett. 2022, 24. doi: 10.1021/acs.orglett.2c00141.
2,2'-Bipyridine-α,α'-trifluoromethyl-diol ligand: synthesis and application in the asymmetric Et2Zn alkylation of aldehydes
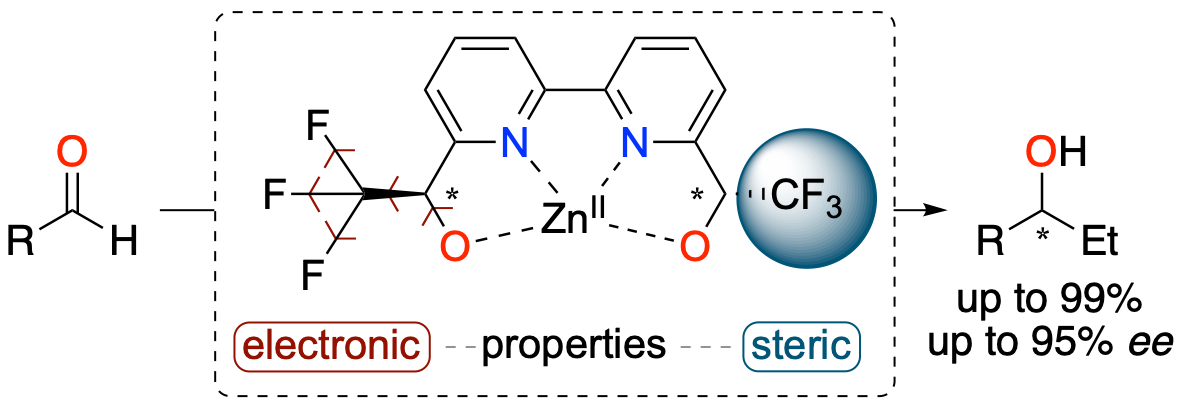
Lauzon, S.; Ollevier, T. Chem. Commun. 2021, 57, 11025–11028. doi: 10.1039/d1cc04556c.
Iron catalysis
Efficient stereoselective synthesis of chiral 3,3'- dimethyl-(2,2'-bipyridine)-diol ligand and applications in FeII-catalysis
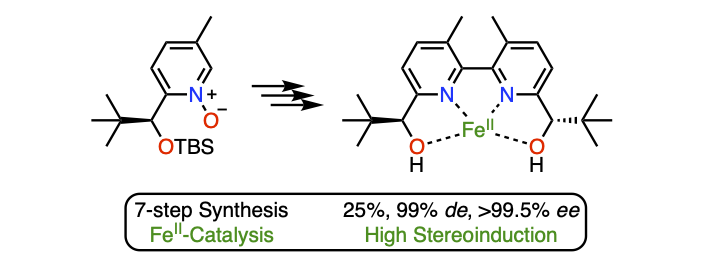
Lauzon, S.; Caron, L.; Ollevier, T. Org. Chem. Front. 2021, 8, 2242–2249. doi: 10.1039/d1qo00188d.
Asymmetric FeII-Catalyzed Thia-Michael Addition Reaction to α,β-Unsaturated Oxazolidin-2-one Derivatives
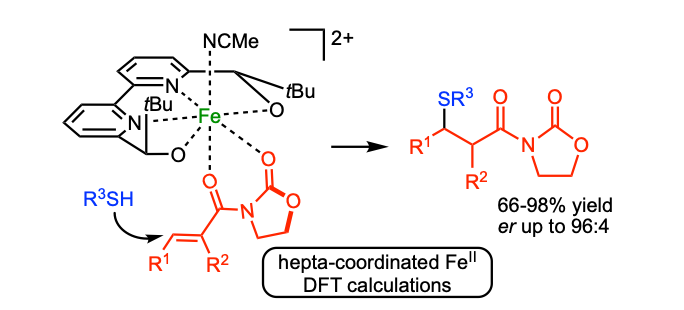
Lauzon, S.; Keipour, H.; Gandon, V.; Ollevier, T. Org. Lett. 2017, 19, 6324–6327. doi: 10.1021/acs.orglett.7b03118. Highlighted in Synfacts:"Iron(II)-Catalyzed Michael Addition" , Lautens, M. ; Zeidan, N. Synfacts 2018, 158.
Asymmetric Diels-Alder Reaction of α,β-Unsaturated Oxazolidin-2-one Derivatives Catalyzed by a Chiral Fe(III)-Bipyridine Diol Complex
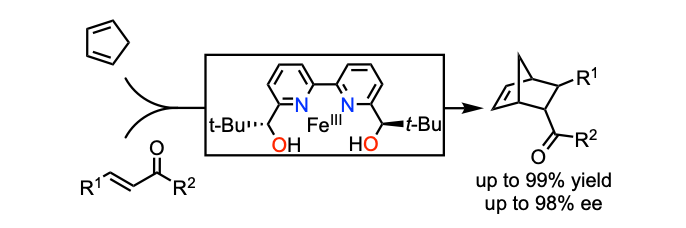
Li, M.; Carreras, V.; Jalba, A.; Ollevier, T. Org. Lett. 2018, 20, 995–998. doi: 10.1021/acs.orglett.7b03939.
Bismuth catalysis
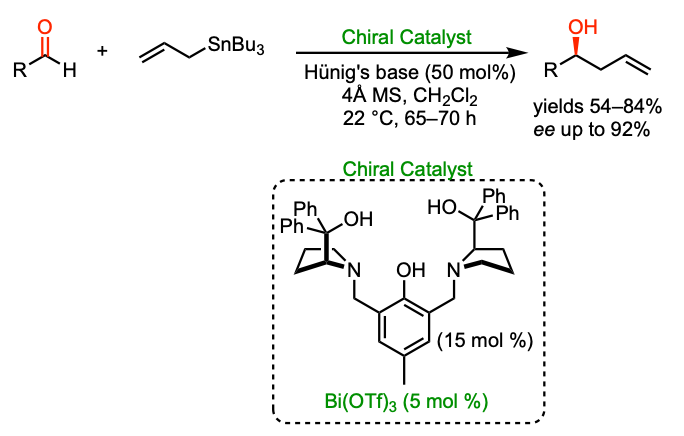
Li, Z.; Plancq., B.; Ollevier, T. "Bismuth Triflate-catalyzed Asymmetric Allylation of Aromatic Aldehydes", Chem. Eur. J. 2012, 18, 3144–3147.
Sulfoxide chemistry
Synthesis of homochiral sulfanyl- and sulfoxide-substituted naphthyltriazoles and study of the conformational stability
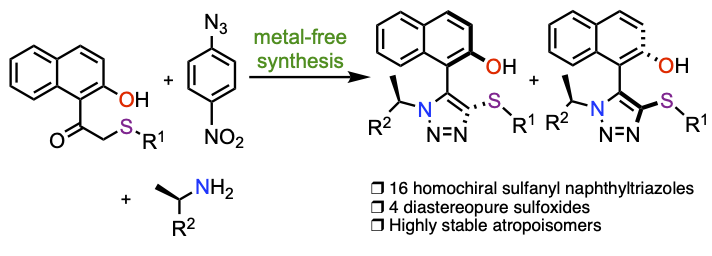
Vroemans, R.; Ribone, S.; Thomas, J.; Van Meervelt, L.; Ollevier, T.; Dehaen, W. Org. Biomol. Chem. 2021, 19, 6521–6526. doi: 10.1039/D1OB00784J.
Enantioselective Aromatic Sulfide Oxidation and Tandem Kinetic Resolution using Aqueous H2O2 and Chiral Iron Bis(oxazolinyl)bipyridine Catalysts
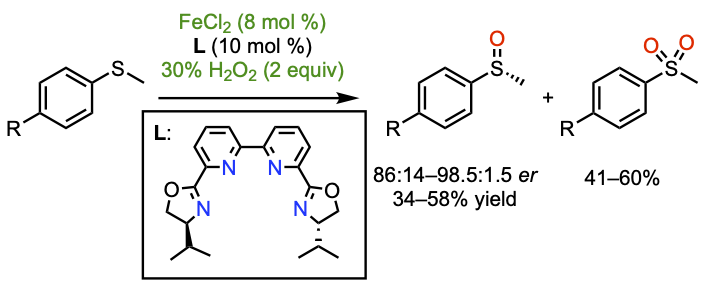
Jalba, A.; Régnier, N.; Ollevier, T. Eur. J. Org. Chem. 2017, 1628–1637. doi: 10.1002/ejoc.201601597.
Electrosynthesis
Anodic oxidation
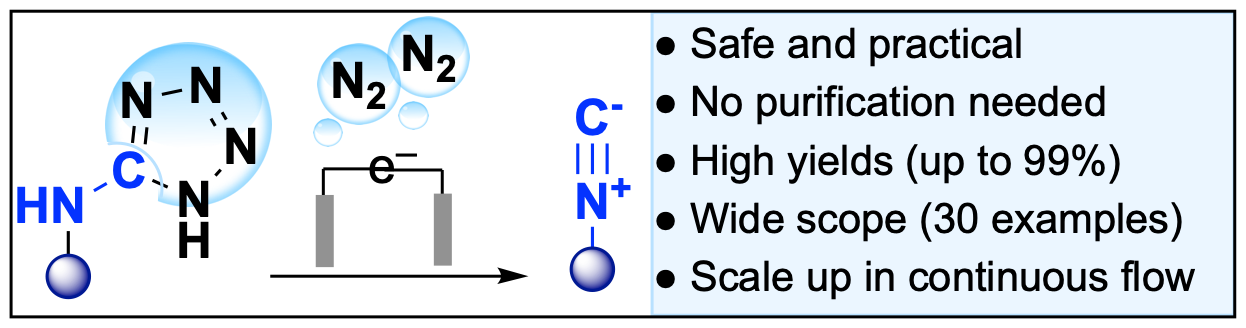
Leech, M. C.; Petti, A.; Tanbouza, N.; Mastrodonato, A.; Goodall, I. C. A.; Ollevier, T.; Dobbs, A.; Lam, K. Org. Lett. 2021, 23, 9371–9375. doi: 10.1021/acs.orglett.1c03475.
Supporting-Electrolyte-Free Anodic Oxidation of Oxamic Acids into Isocyanates: An Expedient Way to Access Ureas, Carbamates, and Thiocarbamates
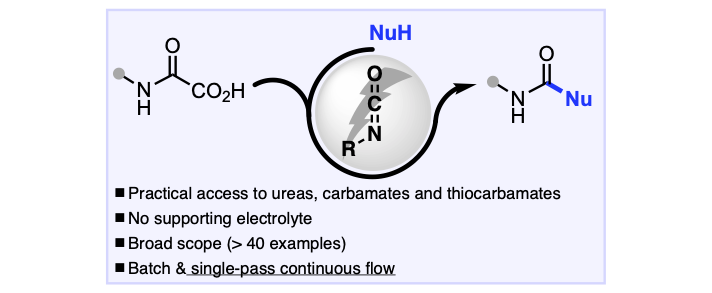
Petti, A.; Fagnan, C.; van Melis, C.; Tanbouza, N.; Garcia, A.; Mastrodonato, A.; Leech, M.; Goodall, I.; Dobbs, A.; Ollevier, T.; Lam, K. Organic Process Research & Development 2021, 25, 2614–2621. doi: 10.1021/acs.oprd.1c00112.
Metal-free chemistry
Hydroamination reactions
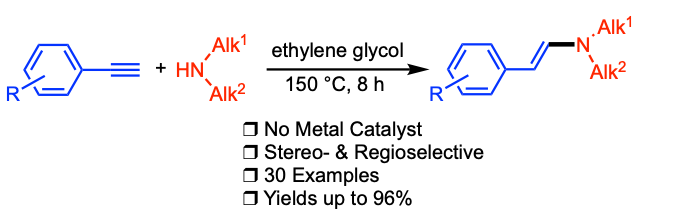
Bahri, J.; Tanbouza, N.; Ollevier, T. Synlett 2019, 30, 2086-2090. doi: 10.1055/s-0039-1690988.
Arylation/vinylation reactions
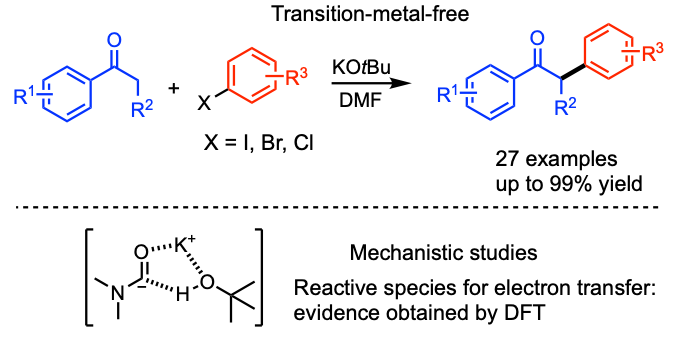
Zaid, Y.; Mboyi, C. D.; Pichette Drapeau, M.; Radal, L.; Ouazzani Chahdi, F; Kandri Rodi, Y; Ollevier, T.; Taillefer, M. Chem. Eur. J. 2018, 24, 17449–17453. doi:10.1002/chem.201804415.
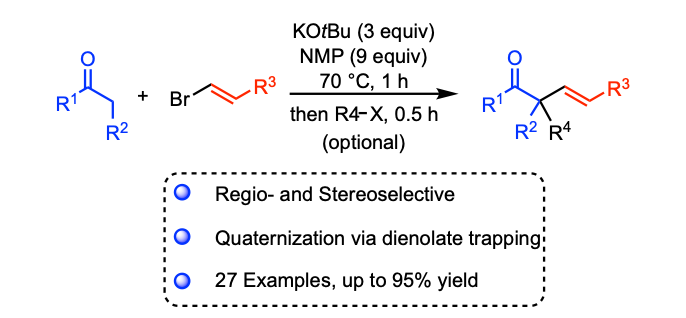
Zaid, Y.; Mboyi, C. D.; Pichette Drapeau, M.; Radal, L.; Ouazzani Chahdi, F; Kandri Rodi, Y; Ollevier, T.; Taillefer, M. Org. Lett. 2019, 21, 1564–1568. doi: 10.1021/acs.orglett.8b04004.
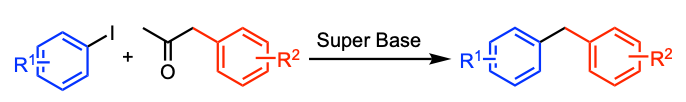
Pichette Drapeau, M.; Tlili, A.; Zaid, Y.; Toummini, D.; Ouazzani Chahdi, F.; Sotiropoulos, J.-M.; Ollevier, T. Chem. Eur. J. 2018, 24, 17449–17453. doi:10.1002/chem.201804415.
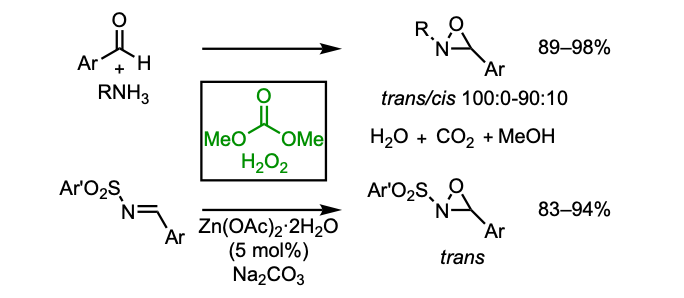
Oxaziridine synthesis
Synthesis of biologically-active molecules
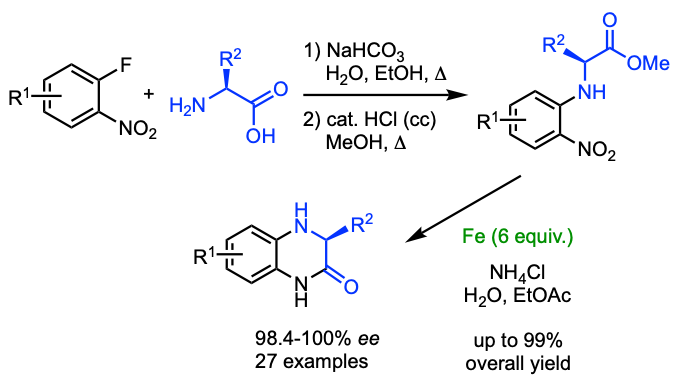
Synthesis of dihydroquinoxazolinones
NHC chemistry
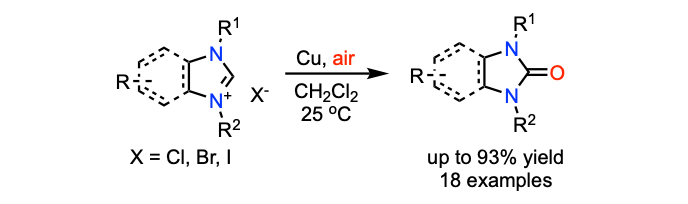
Cu NHC
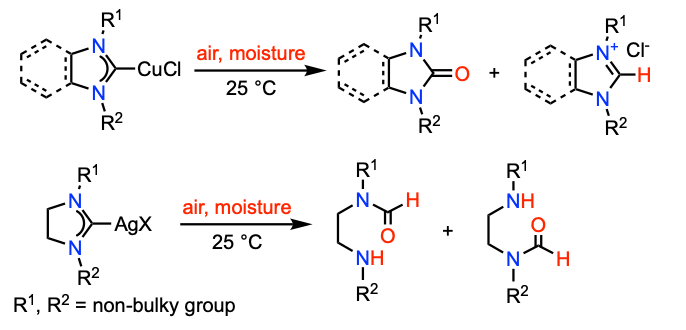
Li, D.; Ollevier, T. J. Organomet. Chem. 2020, 906, 121025. doi: 10.1016/j.jorganchem.2019.121025.
Heterogeneous catalysis
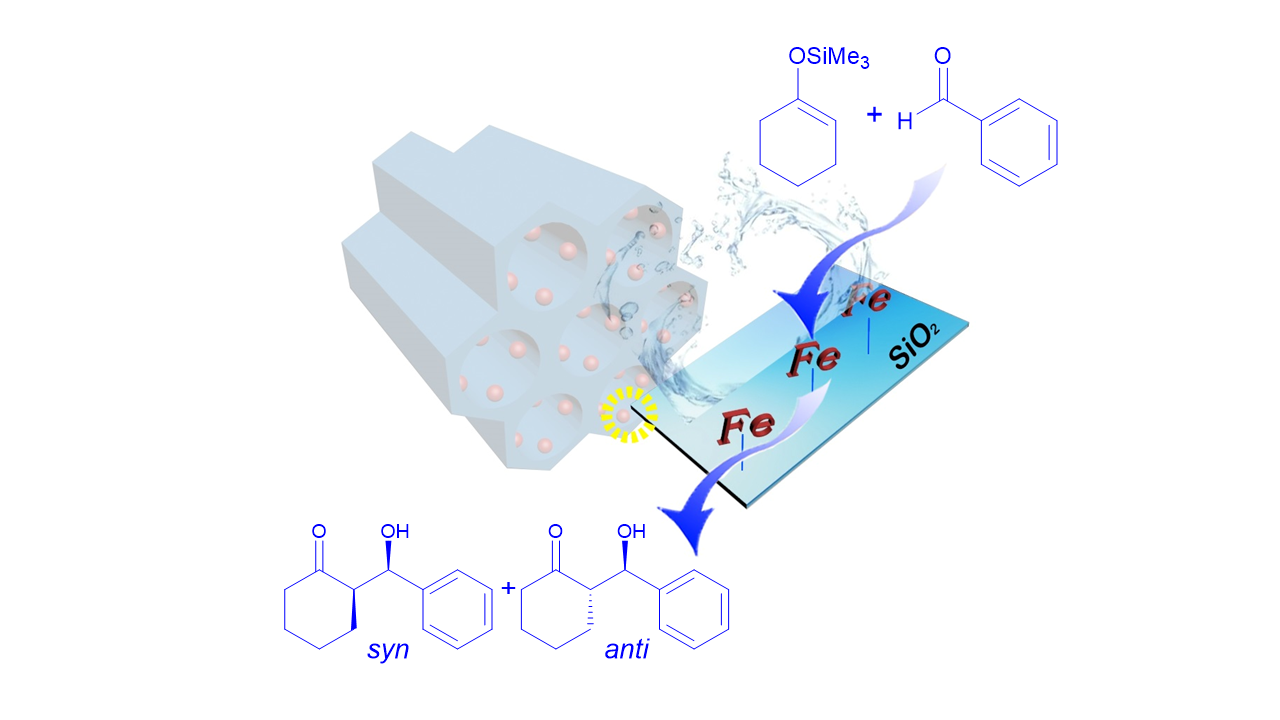
Iron-catalyzed Mukaiyama aldol reaction
Green engineering
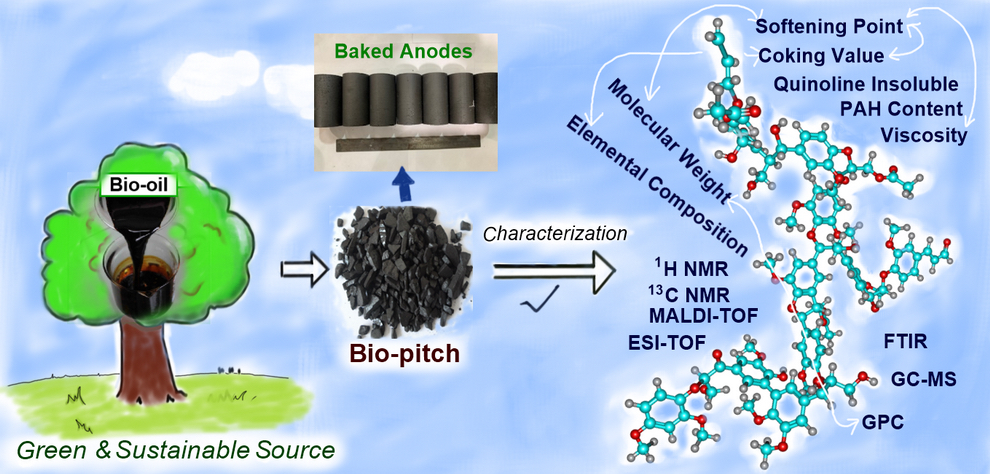
Valorization of biomass
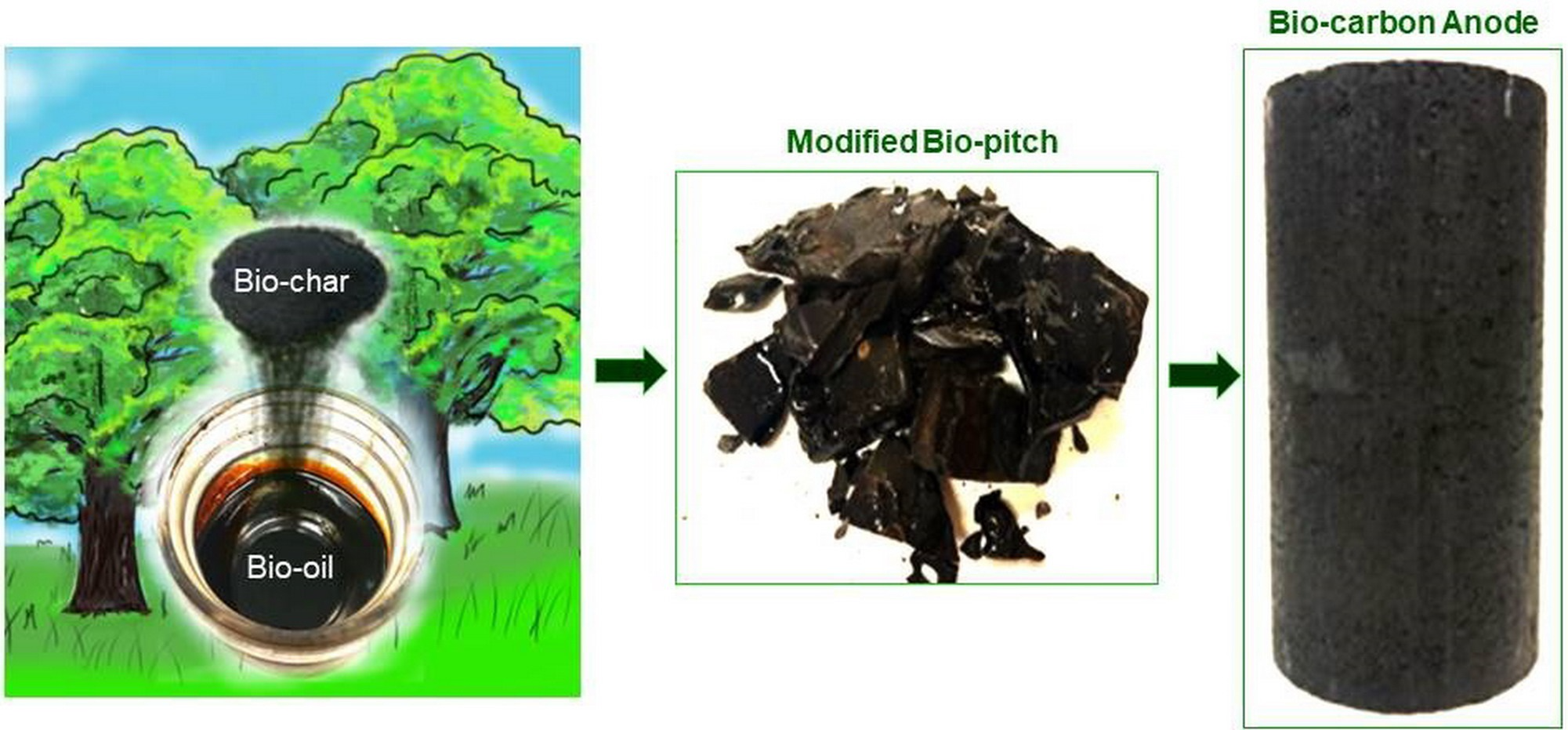
Lu, Y.; Hussein, A.; Lauzon-Gauthier, J.; Ollevier, T.; Alamdari, H. ACS Sustainable Chem. Eng. 2021, 9. doi: 10.1021/acssuschemeng.1c04941.
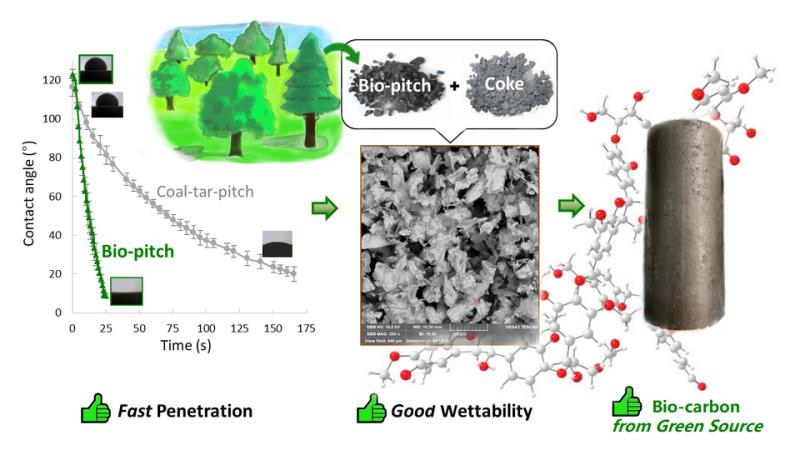
Lu, Y.; Hussein, A.; Li, D.; Huang, X.; Mollaabbasi, R.; Picard, D.; Ollevier, T.; Alamdari, H. ACS Sustainable Chem. Eng. 2020, 8, 15366–15374.