Peptide Science, 2019, 111:e24051.
Lipid membrane interactions of a fluorinated peptide with potential ion channel-forming ability
Maud Auger, Thierry LefÈvre, FranÇois Otis, Normand Voyer, MichÈle Auger
Abstract
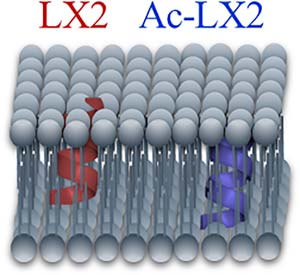
Fluorinated peptides attract much interest in the biomedical area because they generally exhibit an enhanced stability compared to their hydrogenated counterparts and because fluorine atoms represent efficient probes to investigate peptide assemblies, especially in membranes. We previously designed and characterized a fluorinated peptide intended to form ion channels in membranes. This peptide, designated as LX2, adopts a predominantly α‐helical structure and acts as a selective ion channel for various cations. Molecular dynamics indicated that the peptide tetrameric form would be favored. However, the interactions of LX2 with model membranes have not been studied experimentally. Here we investigated the interactions of LX2 and its acetylated form, Ac‐LX2, with eukaryotic model membranes using CD and infrared spectroscopy, as well as 19F, 2H, and 31P NMR spectroscopy. 19F NMR results indicate that the peptides undergo restrained motions in the presence of membranes, which strongly suggests their insertion in membranes. Both LX2 and Ac‐LX2 adopt a well‐defined α‐helical structure, in contrast with the secondary structure observed in hexafluoroisopropanol. The alterations of lipid organization due to LX2 peptides appear overall relatively small but the results suggest that the two peptides are located in the membrane hydrophobic core, LX2 being closer to the interfacial region than Ac‐LX2.