Biochimica et biophysica acta (BBA) - biomembranes , 2021,1863, 183605.
Membrane binding properties of the C-terminal segment of retinol
dehydrogenase 8
AndrÉ HÄdicke, Ana Coutinho, Sarah Roy, FranÇois Otis, Mustapha Lhor, Line Cantin, Manuel Prieto, Normand Voyer, Christian Salesse
Abstract
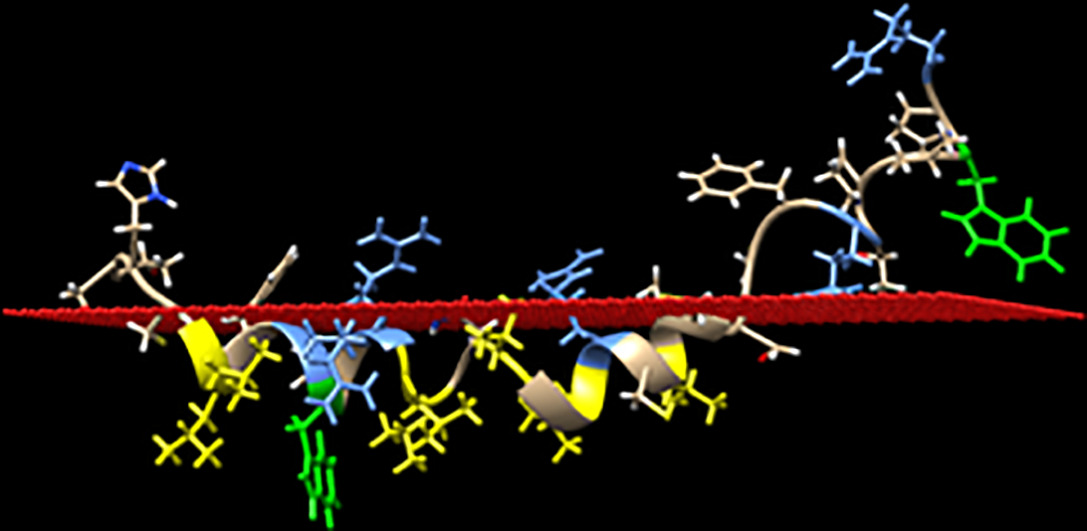
Light absorption by rhodopsin leads to the release of all-trans retinal (ATRal) in the lipid phase of photoreceptor disc membranes. Retinol dehydrogenase 8 (RDH8) then reduces ATRal into all-trans retinol, which is the first step of the visual cycle. The membrane binding of RDH8 has been postulated to be mediated by one or more palmitoylated cysteines located in its C-terminus. Different peptide variants of the C-terminus of RDH8 were thus used to obtain information on the mechanism of membrane binding of this enzyme. Steady-state and timeresolved fluorescence measurements were performed using short and long C-terminal segments of bovine RDH8, comprising one or two tryptophan residues. The data demonstrate that the amphipathic alpha helical structure of the first portion of the C-terminus of RDH8 strongly contributes to its membrane binding, which is also favored by palmitoylation of at least one of the cysteines located in the last portion of the C-terminus.