Synlett, 2023.
Synthesis of lincosamide analogues via oxime resin aminolysis
Thomas Tremblay, PÉnÉlope Haguette, Gabrielle Robert-Scott, Jessica B. AlcÉe, Christopher BÉrubÉ, Catherine Bergeron, Normand Voyer et Denis GiguÈre
Abstract
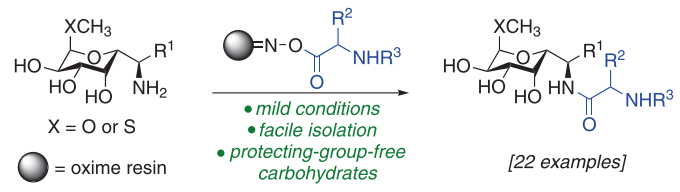
In this work, the synthetic development of an oxime resin aminolysis to lincosamide analogues is described. This synthetic endeavor hinges on a protecting-group-free strategy of the amino sugar nucleophiles. The cleavage from the solid support is achieved under mild conditions in a buffer solution and allows the preparation of a wide diversity of amino acid moieties onto glycosylamine scaffolds. The strategy is further exploited using methylthiolincosamine to generate rapidly complex lincomycin analogues. The results pave the way to access efficiently novel potentially relevant antibacterial compounds.