JOurnal of natural products , 2020, 83, 1778-1783.
Total Synthesis and Antimalarial Activity of Dominicin, a Cyclic Octapeptide from a Marine Sponge
Christopher Bérubé, Alexandre Borgia, Dominic Gagnon, Angana Mukherjee, Dave Richard, and Normand Voyer
Abstract
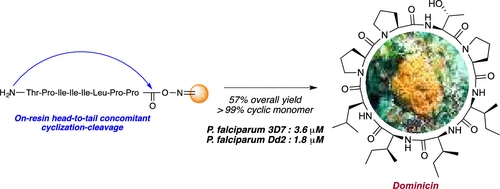
Dominicin, a macrocyclic peptide isolated from the marine sponge Eurypon laughlini, has been synthesized for the first time by solid-phase peptide synthesis. The strategy uses oxime resin and takes advantage of the nucleophile susceptibility of the oxime ester bond. The synthesis relies on the preparation of a linear precursor followed by on-resin head-to-tail concomitant cyclization−cleavage. This is the first report of the use of a Boc/OtBu biorthogonal protection strategy on oxime resin to facilitate concomitant N-terminal and side-chain tert-butyl ether deprotection cyclization of unprotected peptides. Also, we report the first antimalarial investigation of dominicin. Interestingly, the natural macrocyclic peptide demonstrates effective low micromolar activity (1.8 μM) against the chloroquine-mefloquine-pyrimethamine-resistant Dd2 strain of Plasmodium falciparum.